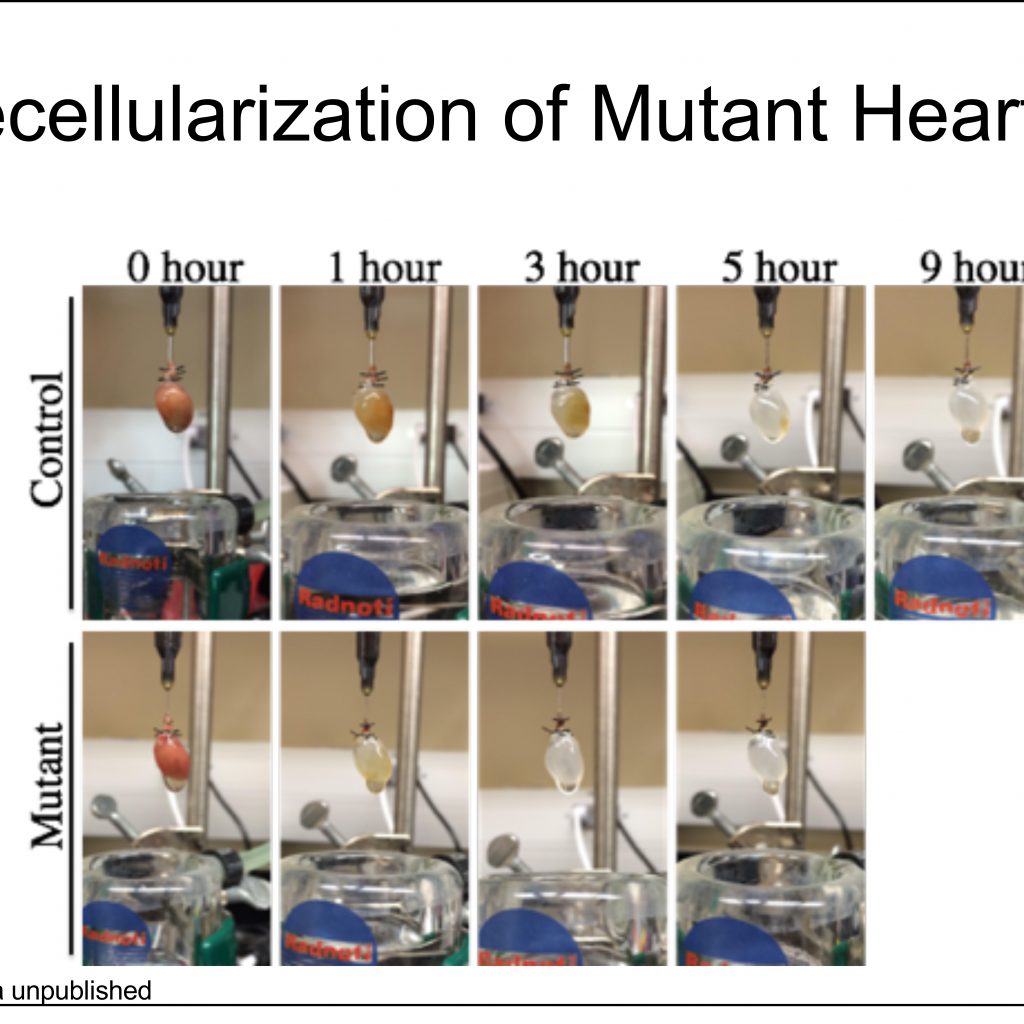
Michelle Tallquist, Ph.D.
Professor of Medicine
Director, Cell & Molecular Biology Graduate Program
Center for Cardiovascular Research
John A. Burns School of Medicine
651 Ilalo Street, BSB 311
Honolulu, HI 96813
Email: michelle.tallquist@hawaii.edu
Phone: (808) 692-1579
The Tallquist Lab is committed to implementing innovative and interdisciplinary approaches in developmental biology, cell physiology, biochemistry, and genetics to repair and regenerate heart and lung tissue in children and adults.
Cardiac and pulmonary microenvironments are the major research focus of my team, particularly processes that shape the vasculature, extracellular matrix, and inflammation, thereby, impacting development and repair mechanisms. Extracellular matrix (ECM) is scaffolding generated by fibroblasts that supports tissues and coordinates cell behavior. In developing organs ECM can regulate diverse cellular activities such as cell proliferation, differentiation, and migration, while in the aged or injured heart, fibroblast activation and ECM production can result in tissue stiffness and reduced function. Despite the importance of ECM, little is understood regarding its role in basic organ physiology. The goal of our research is to understand the mesenchymal cells responsible for ECM production and how changes in ECM impact normal and diseased heart and lung function.
2. Analysis of Tcf21 Gene Function
Although Tcf21, a bHLH transcription factor, expression in the epicardium of the heart was well established, our studies were the first to identify a role for Tcf21 as a gene that is uniquely required for lineage specification of epicardial-derived cells. We found that loss of Tcf21 leads to a deficiency in epicardial-derived cardiac fibroblasts and intriguingly, that vascular smooth muscle cell development proceeds normally (Acharya et al 2012). This was the first identification of a transcription factor required for cardiac fibroblast development. This finding establishes that fibroblast differentiation is instructed and not stochastic. We also developed and implemented an inducible Tcf21-Cre line that enables lineage tracing and genetic manipulation of fibroblast cells in the mouse (Acharya et al 2011). The capacity for fibroblast lineage tracing enabled groundbreaking regeneration studies demonstrating that fibroblasts could be reprogrammed into cardiomyocytes with low efficiency (Song et al 2012). Recently, variants at the Tcf21 locus have been associated with coronary artery disease in several genome wide association studies. In a collaboration, we identified Tcf21 cells in the fibrous cap and demonstrated that Tcf21 expression is correlated with proliferation in the disease process of atherosclerosis (Nurnberg et al 2015). More recently, this collaboration also found that Tcf21 reinforces a fibroblast phenotype during atherosclerosis. Our future work will focus on the role of Tcf21 transcriptional targets in mature cardiac fibroblasts, and its role in lung development. We have exciting data demonstrating a requirement for Tcf21 in lipofibroblasts which control alveologenesis. These studies have the potential to translate to better treatments for bronchopulmonary dysplasia and respiratory distress syndrome.
Our future work will focus on the role of Tcf21 transcriptional targets in mature cardiac fibroblasts, and its role in lung development. We have exciting data demonstrating a requirement for Tcf21 in lipofibroblasts which control alveologenesis. These studies have the potential to translate to better treatments for bronchopulmonary dysplasia and respiratory distress syndrome.
3. Epicardial Development
My lab has contributed to our understanding of how epicardial cell fate is determined and the signaling processes dictating this epithelial to mesenchymal transition (EMT). Epicardial contribution to mesenchymal cells was identified many years ago, but my lab
has been instrumental in the dissection of signaling pathways involved in these complex processes. Using genetic mouse models and in vitro culture systems we have described signal pathways including PDGF receptors, Nf1, Tcf21, and beta-catenin that regulate epicardial differentiation (Mellgren et al 2008; Baek and Tallquist 2012, Wu et al 2010; and Vicente-Steijn et al 2015). Of particular note, we found that PDGFR-alpha signaling was required for EMT of coronary vascular smooth muscle cells and that PDGFR-alpha was required for cardiac fibroblast EMT. This established a unique requirement for each of the two PDGF receptors in promotion of distinct mesenchymal cell populations.
Because recent work has suggested that the epicardium plays positive roles in cardiomyocyte maturation, we are exploring how progenitor cells from the epicardium undergo cell fate choices and determining how these cells facilitate heart development. These results will impact our understanding of smooth muscle cell and fibroblast differentiation and will be advantageous in improving procedures in tissue regeneration and repair.
4. PDGF Receptors in Embryonic Development
My lab has a longstanding interest in the functions of PDGF receptor signaling with an emphasis on congenital birth defects. We have investigated the individual and combined role of these receptors in vertebral abnormalities, neural crest cell defects and extraembryonic vascular defects. These studies have provided valuable data regarding how these receptors shape embryonic development (Richarte et al 2007; Pickett et al 2008; French et al 2008, Chabra et al 2012, and Smith et al 2011.) More recently, we have found that tissue resident fibroblasts require PDGFR-alpha signal transduction for survival (Ivey et al manuscript in preparation). These findings demonstrate that fibroblasts are not quiescent cells residing in the stroma. Instead, they receive constant signals from the surroundings to dictate their survival and proliferation. Future studies will dissect the downstream signaling components and evaluate if similar survival pathways exist in other organ fibroblasts and during disease.
RECENT PUBLICATIONS
Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Zhu K, Chang R, Koyano TK, Fong R, Woo YJ, Liu B, Montgomery SB, Wu JC, Zhu K, Chang R, Alamprese, M, Tallquist MD, Kim JB, Quertermous T. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019 Jul 29. doi: 10.1038/s41591-019-0512-5.
Ivey, MJ, Kuwabara, JT, KL Riggsbee, Tallquist MD. Platelet derived growth factor receptor alpha is essential for cardiac fibroblast survival. AJP-Heart and Circulatory Physiology. 2019;317(2):H330-H344.
Park J, Ivey MJ, Deana Y, Riggsbee KL, Sörensen S, Schwabl V, Sjöberg, C, Hjertberg T, Park GY, Swonger JM, Rosengreen T, Morty RE, Ahlbrecht K, Tallquist MD. The Tcf21 lineage constitutes the lung lipofibroblast population. AJP Lung Cell. Mol. Phys. 2019. May 1;316(5):L872-L885.
Green CD, Ma Q, Manske GL, Shami AN, Zheng X, Marini S, Moritz L, Sultan C, Gurczynski SJ, Moore BB, Tallquist MD, Li JZ, Hammoud SS. A comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-seq. Dev. Cell. 2018; 46:651-667.
Ivey, MJ, Kuwabara, JT, Pai JT, Moore RE, Sun Z, Tallquist MD. Resident fibroblast expansion during cardiac growth and remodeling. JMCC. 2018; 114:161-174.
Kanisicak, O., Khalil, H., Ivey, M.J., Karch, J., Maliken, B.D., Correll, R.N., Brody, M.J., Lin, S.-C., Aronow, B., Tallquist, M.D., Molkentin, J. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016; 7; 12260.
McCarthy N, Liu JS, Richarte AM, Eskiocak B, Lovely CB, Tallquist MD, Eberhart JK. Pdgfra and Pdgfrb genetically interact during craniofacial development. Dev. Dyn. 2016; 245 (6);641-52.
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni M, Debuque RJ, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting cardiac cellular composition. Circ Res. 2016; 118 (3):400-9.
Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599-604
Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bhlh transcription factor tcf21 is required for lineage-specific emt of cardiac fibroblast progenitors. Development. 2012;139:2139-2149
Tallquist, MD. Cardiac fibroblast diversity. Annu. Rev. Physiol. 2020. 82. In press.
Tallquist, MD. Cardiac fibroblasts: from origin to injury. Curr. Opin. In Physiol. 2018. 1:75-79.
Tallquist MD and Molkentin J. Redefining the identity of the cardiac fibroblast. Nature Reviews Cardiology; 2017. 2017. 14(8):484-491.
Kuwabara, JT, Tallquist MD. Tracking adventitial fibroblast contribution to disease: a review of current methods to identify resident fibroblasts. ATVB. 2017. 37(9) 1598-1607.
Ivey MJ, Tallquist MD. Defining the cardiac fibroblast. Circ. J. 2016; 80 (11) 2269-2276.
Swonger JM, Liu JS, Ivey MJ, Tallquist MD. Genetic tools for identifying and manipulating fibroblasts in the mouse. Differentiation. 2016; 92 (3) 66-83.
Tallquist MD. Developmental Pathways of Cardiac Fibroblasts in Heart Development and Disease. Cold Spring Harbor Press. 2019. Eds. Benoit Bruneau and Paul Riley. In press.
Park, J. Tallquist MD. Cardiac Fibroblast in Encyclopedia of Cardiovascular Research and Medicine. 2018:420-433.
Dr. Tallquist’s full list of publications can be found below:
https://www.ncbi.nlm.nih.gov/myncbi/michelle.tallquist.1/bibliography/public/